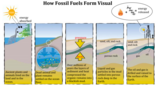
Fossil Fuel Formation | WorksheetFossil Fuel Formation Answer Key
- Subject:
- Environmental Science
- Material Type:
- Homework/Assignment
- Lesson
- Author:
- Sara Catanese
- Date Added:
- 03/11/2021

Fossil Fuel Formation | WorksheetFossil Fuel Formation Answer Key

Learn how friction causes a material to heat up and melt. Rub two objects together and they heat up. When one reaches the melting temperature, particles break free as the material melts away.

Learn how friction causes a material to heat up and melt. Rub two objects together and they heat up. When one reaches the melting temperature, particles break free as the material melts away. Arabic Language.
In a multi-week experiment, student groups gather data from the photobioreactors that they build to investigate growth conditions that make algae thrive best. Using plastic soda bottles, pond water and fish tank aerators, they vary the amount of carbon dioxide (or nutrients or sunlight, as an extension) available to the microalgae. They compare growth in aerated vs. non-aerated conditions. They measure growth by comparing the color of their algae cultures in the bottles to a color indicator scale. Then they graph and analyze the collected data to see which had the fastest growth. Students learn how plants biorecycle carbon dioxide into organic carbon (part of the carbon cycle) and how engineers apply their understanding of this process to maximize biofuel production.
Students learn the components of the rock cycle and how rocks can change over time under the influence of weathering, erosion, pressure and heat. They learn about geotechnical engineering and the role these engineers play in the development of an area of land, the design and placement of new structures, and detection of natural disasters.

The nitrogen cycle game helps you learn how nitrogen atoms move through various forms including soil, the atmosphere, plants and animals. Actions such as lightening, bacteria digestion, plant assimilation, plant death, animal death, herbivorism and nitrogen fixing plant bacteria move nitrogen from one form to another.

Through five lessons, students are introduced to all facets of the rock cycle. Topics include rock and mineral types, material stresses and weathering, geologic time and fossil formation, the Earth's crust and tectonic plates, and soil formation and composition. Lessons are presented in the context of the related impact on humans in the form of roadway and tunnel design and construction, natural disasters, environmental site assessment for building structures, and measurement instrumentation and tools. Hands-on activities include experiencing tensional, compressional and shear material stress by using only hand force to break bars of soap; preparing Jeopardy-type trivia questions/answers for a class game that reinforces students' understanding of rocks and the rock cycle; creating "fossils" using melted chocolate; working within design constraints to design and build a model tunnel through a clay mountain; and soil sampling by creating tools, obtaining soil cores, documenting a soil profile log, and analyzing the findings to make engineering predictions.

Students reinforce their understanding of rocks, the rock cycle, and geotechnical engineering by playing a trivia game. They work in groups to prepare Jeopardy-type trivia questions (answers) and compete against each other to demonstrate their knowledge of rocks and engineering.
Rocks cover the earth's surface, including what is below or near human-made structures. With rocks everywhere, breaking rocks can be hazardous and potentially disastrous to people. Students are introduced to three types of material stress related to rocks: compressional, torsional and shear. They learn about rock types (sedimentary, igneous and metamorphic), and about the occurrence of stresses and weathering in nature, including physical, chemical and biological weathering.

Add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Compare the number of ions in solution for highly soluble NaCl to other slightly soluble salts. Relate the charges on ions to the number of ions in the formula of a salt. Calculate Ksp values.

Add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Compare the number of ions in solution for highly soluble NaCl to other slightly soluble salts. Relate the charges on ions to the number of ions in the formula of a salt. Calculate Ksp values. Arabic Language.
Students learn the basics about soil, including its formation, characteristics and importance. They are also introduced to soil profiles and how engineers conduct site investigations to learn about soil quality for development, contamination transport, and assessing the general environmental health of an area.
Understanding the hazards of space weather on crewed and robotic missions is vital to informing plans for NASA's Journey to Mars and other missions into our solar system, and beyond.