Students will understand how to use the solubility equilibrium constant to calculate the solubility of compounds
- Subject:
- Applied Science
- Material Type:
- Lecture
- Lesson Plan
- Date Added:
- 06/23/2017

Students will understand how to use the solubility equilibrium constant to calculate the solubility of compounds
Students will understand factors that affect the solubility of solutions.
Students will understand how to calculate the equilibrium quotient to determine the direction of solubility and solid formation.

The PhET project at the University of Colorado creates "fun, interactive, research-based simulations of physical phenomena." This particular one deals with Beer's Law. "The thicker the glass, the darker the brew, the less the light that passes through." Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The simulation is also paired with a teachers' guide and related resources from PhET. The simulation is also available in multiple languages.

Biology is designed for multi-semester biology courses for science majors. It is grounded on an evolutionary basis and includes exciting features that highlight careers in the biological sciences and everyday applications of the concepts at hand. To meet the needs of today’s instructors and students, some content has been strategically condensed while maintaining the overall scope and coverage of traditional texts for this course. Instructors can customize the book, adapting it to the approach that works best in their classroom. Biology also includes an innovative art program that incorporates critical thinking and clicker questions to help students understand—and apply—key concepts.

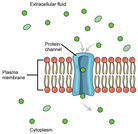
By the end of this section, you will be able to:Explain why and how passive transport occursUnderstand the processes of osmosis and diffusionDefine tonicity and describe its relevance to passive transport

Students learn about various crystals, such as kidney stones, within the human body. They also learn about how crystals grow and ways to inhibit their growth. They also learn how researchers such as chemical engineers design drugs with the intent to inhibit crystal growth for medical treatment purposes and the factors they face when attempting to implement their designs. A day before presenting this lesson to students, conduct the associated activity, Rock Candy Your Body.
We can combine our knowledge of acids and bases, equilibrium, and neutralization reactions to understand buffers and titrations. Solubility equilibria will build on concepts from solubility, precipitation, and equilibrium.

This collection of videos, animations and documents comes from the NCSSM AP chemistry online course. Chapter fourteen provides practice and demonstrations related to gas phase, solubility, and complex ion equilibria in chemistry.

Watch your solution change color as you mix chemicals with water. Then check molarity with the concentration meter. What are all the ways you can change the concentration of your solution? Switch solutes to compare different chemicals and find out how concentrated you can go before you hit saturation!

Students develop and conduct an experiment using the law of conservation of mass to determine whether or not gum should be considered food. Students will compare the mass swallowed for sugar and sugar-free gum. This could be used to discuss solubility.

In this capstone course students will use new and previous knowledge about drug delivery to design capstone innovation project. Throughout the course students will engage in learning opportunities in drug delivery, gain a better understanding of anatomy and physiology related to drug delivery, and participate in a self-directed project to solve a problem. This learning tool will guide students through the process of understanding drug delivery and how drug delivery is applied to treating infectious diseases. DDF’s capstone project is aligned with NGSS and Common Core standards in math and ELA core curriculum. The learning activities, final project, and mid-unit assessments are provided to the teacher and students in the form of eLearning readings, quizzes, interactive tools, and presentation outlines. Students using this module should find success in self-directed learning, though the of use additional resources in the community, at school, at DDF, and in scientific literature, may help them achieve their goal.For more information about expanded learning opportunities, questions about the program, and assistance with learning tools, please contact our DDF eLearning Project Manager Lindsay Malcolm: lmalcolm@tsrlinc.com
This lesson focuses on how food packages are designed and made. Students will learn three of the main functions of a food package. They will learn what is necessary of the design and materials of a package to keep food clean, protect or aid in the physical and chemical changes that can take place in a food, and identify a food appealingly. Then, in the associated activity, the students will have the opportunity to become packaging engineers by designing and building their own food package for a particular type of food.
Students are challenged to think as biomedical engineers and brainstorm ways to administer medication to a patient who is unable to swallow. They learn about the advantages and disadvantages of current drug delivery methods—oral, injection, topical, inhalation and suppository—and pharmaceutical design considerations, including toxicity, efficacy, size, solubility/bioavailability and drug release duration. They apply their prior knowledge about human anatomy, the circulatory system, polymers, crystals and stoichiometry to real-world biomedical applications. A Microsoft® PowerPoint® presentation and worksheets are provided. This lesson prepares students for the associated activity in which they create and test large-size drug encapsulation prototypes to provide the desired delayed release and duration timing.

Students learn how crystallization and inhibition occur by examining calcium oxalate crystals with and without inhibitors that are capable of altering crystallization. Kidney stones are composed of calcium oxalate crystals, and engineers and doctors experiment with these crystals to determine how growth is affected when a potential drug is introduced. Students play the role of engineers by trying to determine which inhibitor would be the best for blocking crystallization.

What determines the concentration of a solution? Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Change solutes to compare different chemical compounds in water.
This lesson will allow students to explore an important role of environmental engineers: cleaning the environment. Students will learn details about the Exxon Valdez oil spill, which was one of the most publicized and studied environmental tragedies in history. In the accompanying activity, they will try many "engineered" strategies to clean up their own manufactured oil spill and learn the difficulties of dealing with oil released into our waters.

This hands-on experiment will provide students with an understanding of the issues that surround environmental cleanup. Students will create their own oil spill, try different methods for cleaning it up, and then discuss the merits of each method in terms of effectiveness (cleanliness) and cost. They will be asked to put themselves in the place of both an environmental engineer and an oil company owner who are responsible for the clean-up.