Students will understand how to calculate the equilibrium quotient to determine the direction of solubility and solid formation.
- Subject:
- Applied Science
- Material Type:
- Lecture
- Lesson Plan
- Date Added:
- 06/23/2017

Students will understand how to calculate the equilibrium quotient to determine the direction of solubility and solid formation.

How do strong and weak acids differ? Use lab tools on your computer to find out! Dip the paper or the probe into solution to measure the pH, or put in the electrodes to measure the conductivity. Then see how concentration and strength affect pH. Can a weak acid solution have the same pH as a strong acid solution?
This course is inline with 2nd year engineering students who would like to understand the concepts of thermodynamics.

Play with objects on a teeter totter to learn about balance. Test what you've learned by trying the Balance Challenge game.

Biology is designed for multi-semester biology courses for science majors. It is grounded on an evolutionary basis and includes exciting features that highlight careers in the biological sciences and everyday applications of the concepts at hand. To meet the needs of today’s instructors and students, some content has been strategically condensed while maintaining the overall scope and coverage of traditional texts for this course. Instructors can customize the book, adapting it to the approach that works best in their classroom. Biology also includes an innovative art program that incorporates critical thinking and clicker questions to help students understand—and apply—key concepts.

By the end of this section, you will be able to:Describe the basic types of ecosystems on EarthExplain the methods that ecologists use to study ecosystem structure and dynamicsIdentify the different methods of ecosystem modelingDifferentiate between food chains and food webs and recognize the importance of each

By the end of this section, you will be able to:Describe the basic types of ecosystems on EarthDifferentiate between food chains and food webs and recognize the importance of each

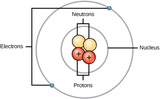
By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms

By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms


This course focuses on feedback control mechanisms that living organisms implement at the molecular level to execute their functions, with emphasis on techniques to design novel systems with prescribed behaviors. Students will learn how biological functions can be understood and designed using notions from feedback control.
We can combine our knowledge of acids and bases, equilibrium, and neutralization reactions to understand buffers and titrations. Solubility equilibria will build on concepts from solubility, precipitation, and equilibrium.
This activity features calculations and rankings as well as student evaluations of their understanding. Instructions are provided on the document, which is ready for distribution to students. It was developed by Celestina A. Pangan and Madhu Gyawali.

This course aims to connect the principles, concepts, and laws/postulates of classical and statistical thermodynamics to applications that require quantitative knowledge of thermodynamic properties from a macroscopic to a molecular level. It covers their basic postulates of classical thermodynamics and their application to transient open and closed systems, criteria of stability and equilibria, as well as constitutive property models of pure materials and mixtures emphasizing molecular-level effects using the formalism of statistical mechanics. Phase and chemical equilibria of multicomponent systems are covered. Applications are emphasized through extensive problem work relating to practical cases.
It discusses the process of equation writing and balancing chemical equations in perspective of the chemical changes that take place during a reaction. This module is the third in a series on chemical reactions.
Bitesize, animated videos covering many chemical reactions topics, organised into these chapters: rates of reactions, equilibrium, redox, electrolysis and energetics through engaging, bitesize animated videos.

This course is a three-part series which explains the basis of the electrical, optical, and magnetic properties of materials including semiconductors, metals, organics, and insulators. We will show how devices are built to take advantage of these properties. This is illustrated with a wide range of devices, placing a strong emphasis on new and emerging technologies.
The first part of the course covers electronic materials and devices, including diodes, bipolar junction transistors, MOSFETs, and semiconductor properties. The second part covers optical materials and devices, including photodetectors, solar cells (photovoltaics), displays, light emitting diodes, lasers, optical fibers, optical communications, and photonic devices. The final part of the series covers magnetic materials and devices, including magnetic data storage, motors, transformers, and spintronics.
This course was organized as a three-part series on MITx by MIT’s Department of Materials Science and Engineering and is now archived on the Open Learning Library, which is free to use. You have the option to sign up and enroll in each modules if you want to track your progress, or you can view and use all the materials without enrolling.