
11 Results

The difference between atomic weight and atomic mass in chemistry, and how it compares to the physics definitions of weight and mass.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Lecture
- Provider:
- Khan Academy
- Author:
- Sal Khan
- Date Added:
- 03/14/2017

How do you know if a chemical equation is balanced? What can you change to balance an equation? Play a game to test your ideas!
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Simulation
- Provider:
- University of Colorado Boulder
- Provider Set:
- PhET Interactive Simulations
- Author:
- Chris Malley
- Emily Moore
- Kathy Perkins
- Kelly Lancaster
- Patricia Loeblein
- Robert Parson
- Date Added:
- 08/15/2011

Biology is designed for multi-semester biology courses for science majors. It is grounded on an evolutionary basis and includes exciting features that highlight careers in the biological sciences and everyday applications of the concepts at hand. To meet the needs of today’s instructors and students, some content has been strategically condensed while maintaining the overall scope and coverage of traditional texts for this course. Instructors can customize the book, adapting it to the approach that works best in their classroom. Biology also includes an innovative art program that incorporates critical thinking and clicker questions to help students understand—and apply—key concepts.
- Subject:
- Biology
- Life Science
- Material Type:
- Full Course
- Provider:
- Rice University
- Provider Set:
- OpenStax College
- Date Added:
- 08/22/2012
- Subject:
- Biology
- Life Science
- Material Type:
- Unit of Study
- Provider:
- Rice University
- Provider Set:
- OpenStax College
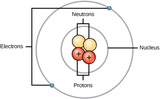
By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Author:
- Tina B. Jones
- Date Added:
- 08/26/2019

By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017

- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017
In this interactive activity, learners build computer models of atoms by adding or removing electrons, protons, and neutrons. It presents the orbital model of an atom: a nucleus consisting of protons and neutrons with electrons surrounding it in regions of high probability called orbitals. Guided tasks are provided, such as constructing a lithium atom and a carbon-12 atom in the fewest possible steps. The activity concludes with a model for building a charged hydrogen atom (an ion). Within each task, students take snapshots of their work product and answer probative questions. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology.
- Subject:
- Applied Science
- Chemistry
- Engineering
- Physical Science
- Physics
- Technology
- Material Type:
- Lesson
- Provider:
- Concord Consortium
- Provider Set:
- Concord Consortium Collection
- Author:
- National Science Foundation
- The Concord Consortium
- Date Added:
- 05/17/2011

Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Simulation
- Provider:
- University of Colorado Boulder
- Provider Set:
- PhET Interactive Simulations
- Author:
- Emily Moore
- John Blanco
- Kathy Perkins
- Kelly Lancaster
- Robert Parson
- Sam Reid
- Trish Loeblein
- Date Added:
- 07/18/2011

Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Simulation
- Provider:
- University of Colorado Boulder
- Provider Set:
- PhET Interactive Simulations
- Author:
- Emily Moore
- John Blanco
- Kathy Perkins
- Kelly Lancaster
- Patricia Loblein
- Robert Parson
- Sam Reid
- Date Added:
- 05/13/2011