Fire as thermal conduction, convection, and radiation.
- Subject:
- Physical Science
- Physics
- Material Type:
- Lesson
- Provider:
- Khan Academy
- Provider Set:
- Khan Academy
- Author:
- Sal Khan
- Date Added:
- 06/01/2021

Fire as thermal conduction, convection, and radiation.
Why metal at room temperature feels cooler than wood at room temperature.

In this experiment, students create a "lava lamp" - a beaker on a hotplate, and investigate buoyancy, convection and other fluid and thermodynamic properties using ink, water, vegetable oil and Alka-Seltzer tablets. The activity is from PUMAS - Practical Uses of Math and Science - a collection of brief examples created by scientists and engineers showing how math and science topics taught in K-12 classes have real world applications.
This lesson includes a powerpoint lecture and problem in-class exercise/assignment with corresponding solutions about thermochemistry.
Doelstelling van dit college is een introductie te geven in de theorie van de Thermodynamica, een van de fundamentele werktuigbouwkunde vakken. De Thermodynamica behandelt energie vraagstukken en relaties tussen de eigenschappen van materialen. In dit college wordt voor een ingenieurs aanpak van de Thermodynamica gekozen: onderwerp van studie zijn systemen en hun interactie met de omgeving. Naast gesloten systemen krijgen open systemen veel aandacht. Thermodynamica wordt, in combinatie met stromingsleer en warmte- en stofoverdracht, ingezet om bijvoorbeeld automotoren, turbines, compressoren, pompen, elektriciteit opwekkinginstallaties, cryogenische-, koel- en klimaat-installaties en duurzame energieconversie installaties te analyseren en ontwerpen. De beginselen van de Thermodynamica maken het mogelijk om de ontwerpen van energie gerelateerde werktuigbouwkundige apparaten en systemen te optimaliseren voor het betreffende doel.
De thermodynamische relaties voor compressibele stoffen en de fasendiagrammen voor pure stoffen worden behandeld. Enkele voorbeelden van toestandsvergelijkingen worden behandeld (de viriaal vergelijking, vergelijkingen met twee constanten en vergelijkingen met meerdere constanten). De grootheden Helmholtz energie en Gibbs energie worden geintroduceerd. De Gibbs vergelijking is de basis om tot een beschrijving van evenwichten (van mengsels) te komen. De condities van evenwicht van pure componenten en van mengsels wordt afgeleidt. Uitgaande van de totale differentialen worden partiele afgeleide uit gedrukt in termen van thermodynamische grootheiden. Voor de berekening van processen, zo als compressie, expansie enz worden uitdrukkingen afgeleid voor delta h, delta s en delta u (waarbij delta voor de deviatie="departure" van ideaal gas gedrag staat). Het wordt getoond hoe deze uitdrukkingen (of dimensieloze diagrammen van deze grootheden) voor de berekening van processen gebruikt kunnen worden. Ook wordt de grootheid exergie gepresenteerd, een grootheid die gebaseerd is op de tweede hoofdwet van de thermodynamica, tezamen met enkele nuttige grootheden en hulpmiddelen voor het uitvoeren van exergie analyses (exergie verlies, exergie rendementen, waardediagram). In dit college wordt de definitie van exergie beperkt tot de thermo-mechanische exergie. Het principe van het stoomturbine kringproces wordt getoond en mogelijkheden voor de optimalisatie van dit kringproces worden besproken, zoals de keuze van stoomdruk en -temperatuur en de toepassing van stoomoververhitting en -herverhitting.
Thermodynamics is the study of heat, "thermo," and work, "dynamics." We will be learning about energy transfer during chemical and physical changes, and how we can predict what kind of changes will occur. Concepts covered in this tutorial include the laws of thermodynamics, internal energy, heat, work, PV diagrams, enthalpy, Hess's law, entropy, and Gibbs free energy.
This lesson is an introductory topic in thermodynamics, on the conversion of energy. The aim of this video is to support students in visualizing the conversion of energy and its importance in real world applications. For this reason, everyday examples are used to help students see the conversion of energy around them. Energy conversion is explored through a simple example of generating electricity for lighting up a shadow puppetry play in a village. The chain process of energy conversion is illustrated until the end product of electricity. This example of electricity generation is further illustrated in an actual industrial setting by taking the viewers to a Power Plant, where viewers will see and hear the explanation of a mechanical engineer on the equipment used to produce electricity that we use in homes and businesses. This important concept of energy conversion is crucial for students to understand as a basis for learning other concepts in Thermodynamics.

This subject deals primarily with equilibrium properties of macroscopic systems, basic thermodynamics, chemical equilibrium of reactions in gas and solution phase, and rates of chemical reactions.
Acknowledgements
The material for 5.60 has evolved over a period of many years, and therefore several faculty members have contributed to the development of the course contents. The following are known to have assisted in preparing the lecture notes available on OpenCourseWare: Emeritus Professors of Chemistry: Robert A. Alberty, Carl W. Garland, Irwin Oppenheim, John S. Waugh. Professors of Chemistry: Moungi Bawendi, John M. Deutch, Robert W. Field, Robert G. Griffin, Keith A. Nelson, Robert J. Silbey, Jeffrey I. Steinfeld. Professor of Bioengineering and Computer Science: Bruce Tidor. Professor of Chemistry, Rice University: James L. Kinsey. Professor of Physics, University of Illinois: Philip W. Phillips.
This subject deals primarily with equilibrium properties of macroscopic systems, basic thermodynamics, chemical equilibrium of reactions in gas and solution phase, and rates of chemical reactions.
Acknowledgements
The material for 5.60 has evolved over a period of many years, and therefore several faculty members have contributed to the development of the course contents. The following are known to have assisted in preparing the lecture notes available on OpenCourseWare: Emeritus Professors of Chemistry: Robert A. Alberty, Carl W. Garland, Irwin Oppenheim, John S. Waugh. Professors of Chemistry: Moungi Bawendi, John M. Deutch, Robert W. Field, Robert G. Griffin, Keith A. Nelson, Robert J. Silbey, Jeffrey I. Steinfeld. Professor of Bioengineering and Computer Science: Bruce Tidor. Professor of Chemistry, Rice University: James L. Kinsey. Professor of Physics, University of Illinois: Philip W. Phillips.

Thermodynamics and Chemistry is designed primarily as a textbook for a one-semester course in classical chemical thermodynamics at the graduate or undergraduate level. It can also serve as a supplementary text and thermodynamics reference source.
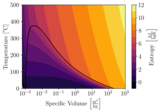
This textbook is intended for a single-semester course in Thermodynamics.The most up-to-date version can be found at Thermodynamics for Engineers (https://github.com/robertbrown2/ThermodynamicsForEngineers). The version as of March 10, 2023 can be found here (www.oercommons.org/editor/documents/13308).It has grown out of the Creative Commons licensed textbook Engineering Thermodynamics - A Graphical Approach by Israel Urieli.

This subject deals primarily with equilibrium properties of macroscopic and microscopic systems, basic thermodynamics, chemical equilibrium of reactions in gas and solution phase, and macromolecular interactions.

Treatment of the laws of thermodynamics and their applications to equilibrium and the properties of materials. Provides a foundation to treat general phenomena in materials science and engineering, including chemical reactions, magnetism, polarizability, and elasticity. Develops relations pertaining to multiphase equilibria as determined by a treatment of solution thermodynamics. Develops graphical constructions that are essential for the interpretation of phase diagrams. Treatment includes electrochemical equilibria and surface thermodynamics. Introduces aspects of statistical thermodynamics as they relate to macroscopic equilibrium phenomena.

Intuition of how gases generate pressure in a container and why pressure x volume is proportional to the combined kinetic energy of the molecules in the volume. Created by Sal Khan.
To begin, Sal solves a constant temperature problem using PV=PV. Then he relates temperature to kinetic energy of a gas. In the second half of the video, he derives the ideal gas law. Created by Sal Khan.

Sal makes the case for the Kelvin scale of temperature and absolute zero by showing that temperature is proportional to kinetic energy. Then he explains that you need to use the Kelvin scale in the ideal gas law. To finish he does a sample ideal gas law problem. Created by Sal Khan.
Sal explains the concept of a mole. Then he derives the molar version of the ideal gas law PV=nRT, where the gas constant R=831 J/molK. Created by Sal Khan.
Sal uses the molar version of the ideal gas law to solve for the number of moles in a gas. He also shows how to convert this answer into number of molecules using Avogadro's number. Created by Sal Khan.

This is a classroom lab investigation where students use the story of the three bears to discover inadequacies in the story and discover how to make them correct. Convection, conduction and radiation are involved.