(View Complete Item Description)
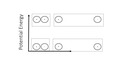
Organic Chemistry research involves the synthesis of organic molecules and the study of their reaction paths, interactions, and applications. Advanced interests include diverse topics such as the development of new synthetic methods for the assembly of complex organic molecules and polymeric materials, organometallic catalysis, organocatalysis, the synthesis of natural and non-natural products with unique biological and physical properties, structure and mechanistic analysis, natural product biosynthesis, theoretical chemistry and molecular modeling, diversity-oriented synthesis, and carbohydrate synthesis.
Material Type:
Textbook