PARylation is crucial to NAT10’s role in the DNA damage response
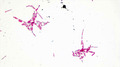
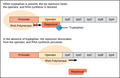
(View Complete Item Description)This resource is a video abstract of a research paper created by Research Square on behalf of its authors. It provides a synopsis that's easy to understand, and can be used to introduce the topics it covers to students, researchers, and the general public. The video's transcript is also provided in full, with a portion provided below for preview: "The protein NAT10 has been implicated in the rapid-aging disease progeria and in human cancers. NAT10 normally resides in the nucleolus, an organelle within the nucleus that assembles cellular protein factories, but when DNA becomes damaged, NAT10 is translocated out of the nucleolus into the thick nuclear fluid, called the nucleoplasm. Researchers recently sought to clarify the mechanism of this translocation using several in vitro experiments. They found that the DNA damage response regulator PARP1 attached ADP-ribose molecules to NAT10 in a process called PARylation. Specifically, PARP1 mediated PARylation at three conserved amino acids within a sequence called the nucleolar localization signal (NuLS). Proper PARylation at these sites was essential for NAT10’s translocation to the nucleoplasm after DNA damage. and for NAT10’s interaction with the chromatin-modifying enzyme MORC2, which helps cells survive after DNA damage..." The rest of the transcript, along with a link to the research itself, is available on the resource itself.
Material Type: Diagram/Illustration, Reading