Students will complete a laboratory experiment, followed by answering questions as to whether the changes were physical or chemical in nature.
- Subject:
- Applied Science
- Material Type:
- Activity/Lab
- Interactive
- Lesson Plan
- Date Added:
- 06/23/2017

Students will complete a laboratory experiment, followed by answering questions as to whether the changes were physical or chemical in nature.

Short Description:
A second semester introductory physics course for life sciences students that looks to deepen students' understanding of biology and chemistry through physics all through the lens of understanding two of the most fundamental particles in the Universe: electrons and photons. The book begins with exploring the quantum mechanical nature of these objects to expand on what students have learned in chemistry and then proceeds to geometric optics (using the human eye as a theme), electrostatics (using membrane potentials), circuits (using the neuron), and finally synthesizing everything in a unit exploring the meaning of "light is an electromagnetic wave."
Long Description:
A second semester introductory physics course for life sciences students that looks to deepen students’ understanding of biology and chemistry through physics all through the lens of understanding two of the most fundamental particles in the Universe: electrons and photons. The book begins with exploring the quantum mechanical nature of these objects to expand on what students have learned in chemistry and then proceeds to geometric optics (using the human eye as a theme), electrostatics (using membrane potentials), circuits (using the neuron), and finally synthesizing everything in a unit exploring the meaning of “light is an electromagnetic wave.”
Word Count: 97595
ISBN: 978-1-945764-07-3
(Note: This resource's metadata has been created automatically by reformatting and/or combining the information that the author initially provided as part of a bulk import process.)

A second semester introductory physics course for life sciences students that looks to deepen students' understanding of biology and chemistry through physics all through the lens of understanding two of the most fundamental particles in the Universe: electrons and photons. The book begins with exploring the quantum mechanical nature of these objects to expand on what students have learned in chemistry and then proceeds to geometric optics (using the human eye as a theme), electrostatics (using membrane potentials), circuits (using the neuron), and finally synthesizing everything in a unit exploring the meaning of "light is an electromagnetic wave."

In this activity, learners construct a device out of a piezoelectric igniter, like those used as barbecue lighters. Learners use the device to remotely start current flowing in a simple series circuit containing a small electric fan.
In a class demonstration, the teacher places different pill types ("chalk" pill, gel pill, and gel tablet) into separate glass beakers of vinegar, representing human stomach acid. After 20-30 minutes, the pills dissolve. Students observe which dissolve the fastest, and discuss the remnants of the various pills. What they learn contributes to their ongoing objective to answer the challenge question presented in lesson 1 of this unit.
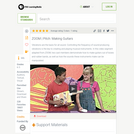
Watch the rubber bands vibrate on homemade guitars in this video segment adapted from ZOOM as cast members talk about pitch and demonstrate how to make a cereal box instrument.
This video segment, adapted from ZOOM, explores the different sounds that a simple drinking straw can produce when you cut the straw and blow into it.
This video segment, adapted from ZOOM, demonstrates how to use a drinking straw and a bottle full of water to make low- and high-pitched sounds.

This is a short unit (including hands on activities) on polymers and plastics to expand our study of physical/chemical properties and changes.

After a brief history of plastics, students look more closely as some examples from the abundant types of plastics found in our day-to-day lives. They are introduced to the mechanical properties of plastics, including their stress-strain relationships, which determine their suitability for different industrial and product applications. These physical properties enable plastics to be fabricated into a wide range of products. Students learn about the different roles that plastics play in our lives, Young's modulus, and the effects that plastics have on our environment. Then students act as industrial engineers, conducting tests to compare different plastics and performing a cost-benefit analysis to determine which are the most cost-effective for a given application, based on their costs and measured physical properties.
This video segment adapted from A Science Odyssey uses animation and archival footage to provide an overview of the theory of plate tectonics.
The distribution of earthquakes and volcanoes around the world confirmed the theory of plate tectonics first proposed by Wegener. These phenomena also help categorize plate boundaries into three different types: convergent, divergent, and transform.
This module offers an introduction to the concepts explored by Alfred Wegener, Harry Hess, and others. It is the first in a series on plate tectonics.

This quiz for younger students asks them 10 questions about plate motions, rock types in continental and oceanic crust, crustal formation and mountain building, the supercontinent Pangea, and the theory of continental drift. A link to a page on continental drift provides information to answer the questions.
This video segment adapted from NOVA uses animation to show the relationship between the movement of a tectonic plate and whether volcanoes on the Hawaiian Islands are active or dormant.

In this activity, learners work in groups to determine the mass and volume of four samples: glass marbles, steel washers or nuts, pieces of pine wood, and pieces of PVC pipe. Learners then plot the data points on a large class graph of mass vs. volume to discover that data points for a particular material form a straight line, the slope of which gives the density of the material.
In this classroom activity, students measure the energy use of various appliances and electronics and calculate how much carbon dioxide (CO2) is released to produce that energy.
This resource will help students learn numerous polyatomic ions utilizing a simple mnemonic.

This course presents the mechanical, optical, and transport properties of polymers with respect to the underlying physics and physical chemistry of polymers in melt, solution, and solid state. Topics include conformation and molecular dimensions of polymer chains in solutions, melts, blends, and block copolymers; an examination of the structure of glassy, crystalline, and rubbery elastic states of polymers; thermodynamics of polymer solutions, blends, crystallization; liquid crystallinity, microphase separation, and self-assembled organic-inorganic nanocomposites. Case studies include relationships between structure and function in technologically important polymeric systems.

This is an activity on polymers and plastics to expand our study of physical/chemical properties and changes.